Post-synthesis deposition of mesoporous niobic acid with improved thermal stability by kinetically controlled sol–gel overcoating
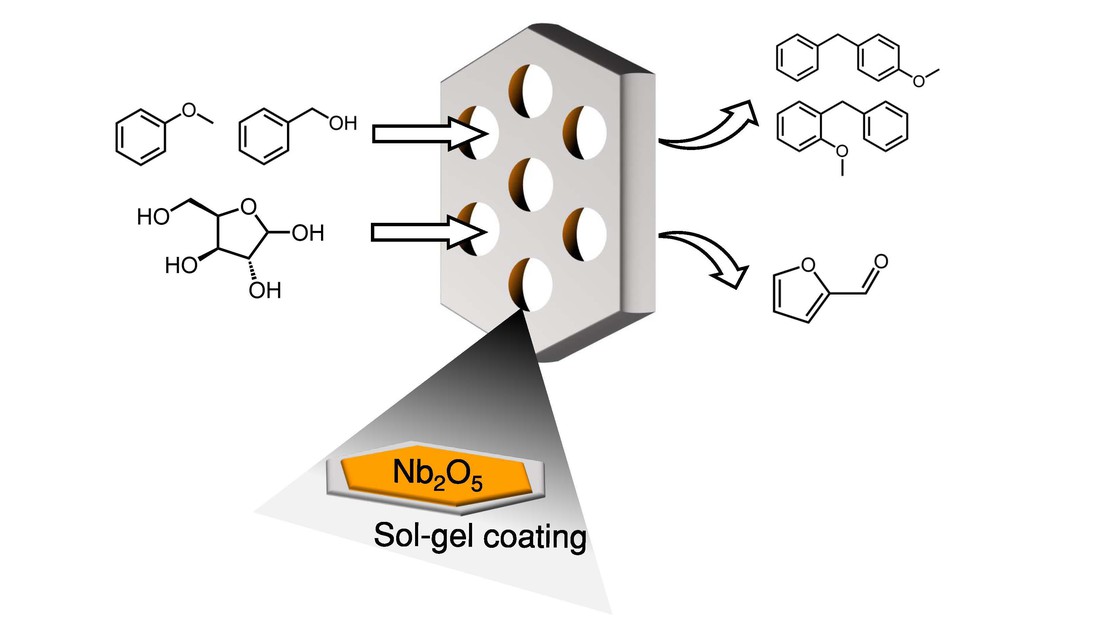
Niobia is a well-known solid acid catalyst owing to its intrinsic Brønsted and Lewis acidity. However, catalyst development with this oxide has been limited by our ability to control its pore structure and thermal stability. Here we report a novel post-synthetic approach for preparing mesoporous niobia catalysts. This method relies on controlling the kinetics of niobium(V) ethoxide to deposit conformal Nb2O5 overcoats on SBA-15 in a typical Stöber solution. Full Nb2O5 coverage over the mesopores of SBA-15 was achieved by adding 4 monolayer equivalents of precursor (4Nb2O5@SBA-15), which was verified by X-ray photoelectron spectroscopy. This overcoated SBA-15 had a high surface area and retained a narrow as well as ordered pore size distribution. Importantly, the typical structural transition from the amorphous to pseudo-hexagonal Nb2O5 phase did not occur with the overcoat after calcination at 773 K. Limiting this crystallization imparts an unprecedented thermal stability to our niobia overcoat, which enables the acid sites to be well preserved after catalyst regeneration. Furthermore, 4Nb2O5@SBA-15 showed higher yields than commercial niobia (HY-340) and lab-synthesized bulk niobia in two probe reactions: xylose dehydration to furfural and Friedel–Crafts alkylation. In both cases, the improvement could be explained by the unique structural features of the niobia overcoat, including a favorable ratio of Brønsted and Lewis acid sites in the case of xylose dehydration and a high proportion of isolated Nb–OH groups for the alkylation reaction. Such structural features and unprecedented thermal stability provide additional tools for synthetizing unique solid acid catalysts using a simple post-synthesis deposition method.